Overall Solution for SARS-CoV-2
Intended use:
The kit is used for in vitro qualitative detection of the ORF1AB gene of the novel coronavirus (2019-NCOV) in throat swab and sputum samples collected from suspected cases of COVID-19, suspected patients with clustered cases, and other individuals who need to make a diagnosis or differential diagnosis of novel coronavirus infections.
Packing Specifications:
24 Tests/Kit, 48 Tests/Kit, 96 Tests/Kit
Product components:
2019-nCoV reaction unit, buffer IV, magnesium acetate, 2019-nCoV positive control, negative control (G)
SARS-CoV-2 Nucleic Acid Detection Kit(Fluorescent RT-RAA)

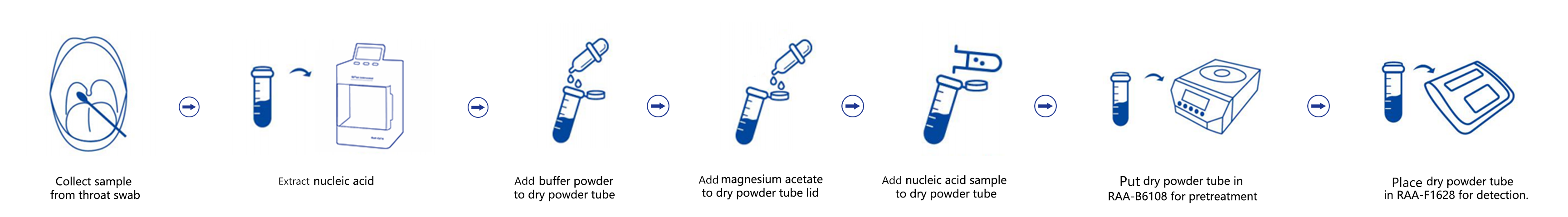
Operation Process

Product Features
Lower Detection Limit: 384 copies/ml
Specificity: no cross-reaction, can detect a variety of mutants
Detection Time: 8-15 min

Storage Conditions And Expiration Date


WeChat Official Account


Service Hotline:
0510-85385531Telephone:
18921157475Address:
4-5F, Building B, Xingye Building, No. 97 Linghu Avenue, Xinwu District, Wuxi CityF